Manufacturing
Clatan has a vast manufacturing platform in India which comprises active pharmaceutical ingredient (API), oral solid dose (OSD) and injectables facilities that serve a number of markets around the world and produce medicines in a wide range of therapeutic areas.
Clatan’s has facilities in India, including all of its API facilities, are approved by the U.S. Food and Drug Administration (FDA). Many of these sites also are approved and regularly inspected by other regulatory authorities, including Australia’s Therapeutic Goods Administration, the U.K.’s Medicines and Healthcare Products Regulatory Agency and the World Health Organization.
Dosage Forms
Syrups
Immediate-release tablets
Sachet filled with powder
Modified-release tablets
Soft gel
Capsules filled with powder and pellets
Injectables
Packaging Capabilities
- Alu-Alu packaging
- Blister packaging
- Blister pouch
- Blister co-pack
- Bottle packaging
Manufacturing Capabilities

Bi-Layered Tablets

Capsules banding

Direct compression

Dry granulation

Extrusion spherization

Fluid bed drier

Hot melt extrusion

Laser drilling
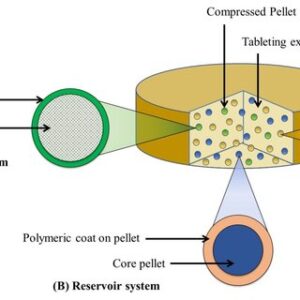
Multiple-unit pellet system

Pellets and beads

Roller compaction

Solid drug layering
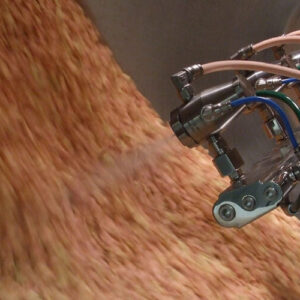
Tablet coater

Wet granulation

Soft gel

Syrups

Injectables

Extrusion spherization

Pellets and beads

Wurster coater with capability to handle solvents

Soft gel

